Can Iron Be Absorbed Through Skin?
Introduction
Iron is a vital co-factor for proteins and enzymes involved in energy metabolism, respiration, Dna synthesis, prison cell bicycle arrest and apoptosis. Over the past x years, major advances have been made in understanding the genetics of fe metabolism and this has led to identification of a number of new proteins, including hepcidin, an acute stage protein that is the main regulator of iron absorption and utilization, often activated in chronic diseases (Weiss, 2009; Finberg, 2013).
Historically, it has long been known that iron is essential for salubrious skin, mucous membranes, hair and nails. Clinical features of atomic number 26 deficiency include skin pallor, pruritus, and predisposition to skin infection (impetigo, boils and candidiasis), angular chelitis, swollen tongue, delicate nails, kolionychia, and dry brittle hair.
Role of Iron in the Skin
Normal Physiology of Atomic number 26
The normal physiology of fe in the skin is complex and not conspicuously understood. Information technology is known that fe levels in normal epidermis are thought to vary over a wide range (Molin and Wester, 1976; Kurz et al., 1987). Inside normal dermis, iron levels besides vary and are thought to increase during the aging procedure (Leveque et al., 2003). Furthermore, atomic number 26-containing proteins accept specific office such as the metabolism of collagen by procollagen-proline dioxygenase (Richardson et al., 1996; Polefka et al., 2012; Figure i). Iron is not actively excreted from the body, notwithstanding the skin is a key organ in iron hemostasis every bit fe is lost through the skin by desquamation (Effigy 2). Current theories regarding the underlying mechanisms of desquamation include active dissolution of desmosomes involved in keratinocyte cell–jail cell adhesion, by hydrolytic protease digestion (Milstone, 2004). Desquamation of keratinocytes is thought to account for 20–25% of captivated iron that is lost (Jacob et al., 1981). Yet overall, the daily loss of iron by desquamation is approximately 25% that of daily urinary iron excretion (Molin and Wester, 1976). Evidence is emerging from genetic model mouse studies by Milstone et al. (2012) that both loss of iron past desquamation and local changes in epidermal iron metabolism accept some role in systemic iron metabolism (these studies investigated three groups of mice: firstly mice overexpressing of HPV16 E7 gene, which causes a threefold increase in epidermal turnover, secondly mice overexpressing the transferrin receptor which causes a iii to fourfold increase of epidermal iron in a peel model, and finally a systemic hemochromatosis knockout model crossed with the epidermal iron sink model). Additionally, gender-related differences in iron status may be responsible for the increased longevity of women as compared to men. The relative difference in cell atomic number 26 levels between the sexes may be of importance both physiologically and in setting of pathophysiological conditions (Pouillot and Polla, 2013).
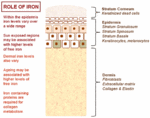
FIGURE i. Office of iron in the skin – an overview.
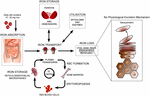
Effigy ii. Normal physiology of iron homeostasis. Figure adapted to evidence primal atomic number 26 physiology including iron loss by desquamation (run across Milstone et al., 2012).
Iron, Oxidative Stress, and Photo-Induced Damage
The main causes of oxidative stress in the skin are reactive oxygen species (ROS) generated in the pare by ultraviolet (UVA) 320–400 nm portion of the UVA spectrum. Atomic number 26 plays a cardinal role in oxidative stress processes, as it is a transition metal, which exists in two stable states, Fe2+ (electron donor) and Fe3+ (electron acceptor). Intracellular labile iron can undergo redox cycling betwixt its well-nigh stable oxidation states (Feii+/Fe3+) and react with ROS such equally superoxide anion, hydrogen peroxide, giving rise to hydroxyl radicals via the Fenton reaction or superoxide-driven Fenton chemistry (Pelle et al., 2011).
Exposure of pare fibroblasts to UVA tin generate ROS that promote oxidative damage in lysosomal, mitrochondrial, nuclear, and plasma membranes. Ultimately loss of plasma membrane integrity together with mitrochondial ATP depletion results in necrotic cell death (Aroun et al., 2012). Information technology is thought that compared with skin fibroblasts, keratinocytes are more resistant to UVA mediated membrane damage and cytotoxicity. In vitro studies have shown that although UVA starts lysosomal impairment, ferritin degradation and cytosolic labile fe release in keratinocytes, the absolute level of UVA induced labile atomic number 26 release is several fold lower than in fibroblasts, suggesting a link between labile iron release and keratinocyte resistance to UVA mediated damage (Zhong et al., 2004).
Anemia, Atomic number 26 Deficiency, and Cutaneous Wound Healing
Wound healing is a dynamic and highly regulated procedure consisting of cellular, humoral and molecular mechanisms (Reinke and Sorg, 2012). The normal cutaneous wound healing process is a temporal procedure involving a complex series of overlapping events, which can exist divided into key stages including: hemorrhage and fibrin-jell formation, inflammatory response, re-epithelialization, granulation tissue formation, angiogenic response, connective tissue contraction, and remodeling, see Figure 3.
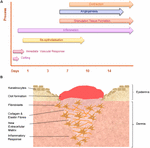
FIGURE 3. The temporal stages of cutaneous wound healing and key cell types involved. (A) Graph illustrating the key temporal stages of wound healing over the 14 days mail injury. (B) The key jail cell types involved in the wound healing process.
Experimental Studies – Fe, Anemia, and Wound Healing
In current literature, beast studies autumn into two distinct groups. Early studies investigating the result of anemia on wound healing used a diverseness of experimental methodology to found anemia or iron deficiency. They focused on wound strength studies rather than initial macroscopic healing or histological studies of re-epithelialization. More than contempo studies have investigated novel treatments aimed at correcting the upshot of systemic iron deficiency and topical application of fe-chelators to reduce iron at the specific site of inflammation and in particular their effect on pro inflammatory macrophages.
In Vivo Studies – the Upshot of Iron Deficiency on Wound Healing
Early initial experimental rodent studies used powdered milk diet to establish iron deficiency. Jacobson and Vanprohaska (1965) constitute that grub-fed command mice showed significantly higher wound breaking-strength than bloodless mice that were fed on an iron-free powdered milk nutrition. Bains et al. (1966) found that young rats fed low iron (powdered milk) diet and subjected to repeated bleeding to produce chronic anemia had weaker wound tensile force. However, later studies undertaken past Macon and Pories (1971) had contrary findings; fe-deficiency anemia (IDA) had no effect on wound breaking strength. This may reflect the methodological bug when using powdered milk to institute iron deficiency as Waterman et al. (1952) showed that control and anemic rats fed powdered milk had slower wound contraction and reduced wound breaking force, when compared with animals fed normal chow.
Investigation into the impact of anemia and claret volume on wound healing strength by Sandberg and Zederfeldt (1960) plant that replacing blood volume with dextran restored normal wound healing, following acute hemorrhage in a rabbit model. Heughan et al. (1974) establish that in that location was no significant alter in wound-fluid oxygen tension (POii) in rabbits made anemic by bleeding and re-transfusing plasma. Additionally, connective tissue weight was greater at lower packed jail cell volumes, an initial finding that suggested a deleterious result of hypoxia on collagen synthesis.
Oliveira Sampaio et al. (2013) investigated the effect of delivery of an iron gratis diet for fifteen days in Wistar rats, though histological study of excisional wounds at 7, fourteen, and 21 days postal service-healing. LED calorie-free caused a significant positive bio-modulation of fibroblastic proliferation in anemic animals, with light amplification by stimulated emission of radiation being more effective on increasing proliferation in non-anemic animals. This report did not describe the effect of fe deficiency on the early stages of wound healing (re-epithelialization) or later resolution.
There are diverse mechanisms by which iron deficiency may impair wound healing. Current evidence favors a fundamental role played by hypoxia. Hypoxia-inducible factor-1 (HIF-1) contributes to all stages of wound healing (through its office in cell migration, cell survival under hypoxic conditions, jail cell division, growth factor release, and matrix synthesis) and positive regulators of HIF-1, such as prolyl-4-hydroxylase inhibitors, have been shown to exist beneficial in enhancing diabetic healing (Hong et al., 2014). Farther studies are required to directly answer this question.
Of note, the functional role of atomic number 26 in the wound healing process has not undergone detailed in vitro study. Recent interest in lactoferrin, an iron-binding glycoprotein secreted from glandular epithelial cells, has focused on its role in promoting cutaneous wound healing by enhancing the initial inflammatory phase, and cell proliferation and migration. Takayama and Aoki (2012) institute using an in vitro model of wound wrinkle, lactoferrin promoted fibroblast-mediated collagen gel contraction.
In Vivo Studies – the Effect of Iron Chelators on Cutaneous Wound Healing
There is considerable variability in iron chelator structure, mechanism of activeness and their consistent applications. The most widely used iron chelator used to treat iron over-load is deferoxamine, see Tabular array 1. Different fe chelators have been applied in studies using a variety of wound healing models.

Table 1. Summary of iron chelators.
Early studies of porcine flap necrosis found that intramuscular injection of deferoxamine decreased the per centum of flap necrosis (Weinstein et al., 1989). This study provided some indirect evidence suggesting that iron chelators have a positive effect on wound healing. Mohammadpour et al. (2013) carried out a wound healing report in Wistar rats, primarily assessing macroscopic wound area calculations at days 4, 8, and 12. They plant that topical deferiprone treatment accelerated macroscopic wound healing more than than Kojic acrid, and on the basis of further DPPH scavenging analysis suggested that this was due to its higher antioxidant and iron chelation abilities.
Iron chelation results in increased VEGF and HIF ane-α and positive effect on angiogenesis The effect of iron chelation on granulation tissue formation and angiogenesis has not been demonstrated in cutaneous wound healing studies, although there take been some studies of bone tissue in the context of fracture healing (Phelps et al., 1986; Farberg et al., 2012; Donneys et al., 2013). Localized Deferoxamine injection has been shown to both reverse radiation induced hypovascularity and broaden vascularity in pathologic fracture healing.
The incorporation of fe chelators in novel wound dressing for human chronic wound handling has too been described. Wenk et al. (2001) suggested that in human being chronic wounds, wound fluid iron-levels are elevated compared with astute wounds. They developed a novel wound dressing based on deferoxamine coupled cellulose and in vitro assays suggested that this dressing may target iron-driven induction of matrix-degrading metalloproteinase-i and lipid peroxidation. Taylor et al. (2005) as well developed and described successful biomechanical testing of deferoxamine coupled polyurethane net substrates.
Clinical Studies – Part of Iron in Human Cutaneous Wound Healing
Human being studies in patients with anemia take focused on wound strength. These studies have involved small case-serial of patients with a variety of acute surgical conditions. Jonsson et al. (1991) performed a study of 33 patients undergoing subcutaneous implantation of ePTFE graft, collagen deposition was directly proportional to wound oxygen tension and measures of perfusion, although the anemia seen in these patients was not fully described. Pavlidis et al. (2001) carried out a retrospective analysis of 89 patients and plant that anemia was not associated with laparotomy wound dehiscence. In a written report of 35 normovolaemic anemic patients undergoing skin grafting, Agarwal et al. (2003) found at that place was no difference in wound healing as assessed by hateful dissever-thickness skin graft take.
To date, human being studies have not demonstrated the specific furnishings of iron deficiency and anemia on the histological stages of chronic wound healing. Clinical studies past our group have institute an association between diabetic foot ulceration (DFU) severity and hemoglobin (Hb) decline. DFU is a complex status, characterized by poor wound healing. Over one-half all severe DFU patients take IDA (Khanbhai et al., 2012). Clinically the anemia is hard to narrate; a significant proportion of patients accept a functional iron deficiency (FID) acquired by chronic inflammation and disruption of the normal Hepcidin mediated fe absorption pathways.
Dysregulation of Local Cutaneous Iron Homeostasis in Chronic Leg Ulceration
Chronic inflammatory atmospheric condition such as rheumatoid arthritis (RA) and Lupus Erythematosus are associated with dysregulation of local cutaneous iron hemostasis.
RA is a progressive inflammatory autoimmune disease, with articulation articular and systemic effects including evolution of ulceration and poor wound healing. The release of cytokines, specially TNF-α, IL-6, and IL-ane, causes synovial inflammation. Pro-inflammatory cytokines also promote the evolution of systemic effects, including production of astute-phase proteins (such equally CRP) which in plow may contribute to development dysregulation of atomic number 26 homeostasis and anemia (Choy, 2012). Indeed, clinical studies of RA patients take reported both iron deficiency anemia and anemia of chronic disease (Bari et al., 2013). Inflammation upregulates the expression of atomic number 26-related proteins in the duodenum and monocytes of RA patients (Sukumaran et al., 2013). Evidence for a role for IL-6 signaling in RA is emerging. Isaacs et al. (2013) found that tocilizumab results in an improvement in anemia, reduction in hepcidin/haptoglobin and increase in iron-binding capacity. Approximately 10% of patients with RA develop leg ulceration.
Clinically, RA leg ulcers are typically associated with venous insufficiency, trauma, arterial insufficiency and rarely vasculitis (for review, meet Rayner et al., 2009). Further work is needed to await at the effect of IAD in patients with RA and leg ulceration.
Lupus Erythematosus is an autoimmune disorder with various clinical manifestation ranging from balmy cutaneous disorder to a life-threatening systemic illness (SLE). Some patients endure from a peel-limited form (with a diversity of manifestations including oral ulceration), while in others it evolves into SLE, although this process is non fully understood. A central exogenous trigger to the onset of cutaneous disease activeness is exposure to UV radiation. It has been shown that photosensitive patients with cutaneous lesions limited anti-Ro/SSA autoantibodies. In vivo studies have demonstrated up-regulation of antigens such as Ro52 in keratinocytes (Oke and Wahren-Herlenius, 2013). This is of some involvement; information technology is possible that iron release in response to UV radiation impairs the function of these antigens, which appear to play a role in negative feedback in response to inflammation.
Deleterious Effects of Local Cutaneous Iron Deposition
There has been some interest in the part of excess iron stored in the skin as hemosiderin, in the pathophysiology of chronic venous affliction (CVD). Information technology is at present thought that the severe pare changes (such every bit lipodermatosclerosis) and leg ulceration associated with CVD happen after fe overload occurs. The mechanisms underlying the deleterious effects of local cutaneous iron deposition in CVD are shown in Figure 4.

Effigy 4. Mechanisms of the deleterious furnishings of local cutaneous iron deposition in CVD. Venous hypertension (characterized by abnormally leaky venous valve) leads to extravasation of erythrocytes with fe. There is increased hemosiderin deposition in the dermis. Macrophages become loaded with iron (by erythrophagocytosis) resulting in unrestrained pro-inflammatory macrophage activation. ROS produced cause a cascade of deleterious reactions and increase oxidative stress. There is further inflammatory response through tumor necrosis cistron alpha (TNF-α) and interleukin-6 (IL -6) which is constantly secreted venous leg ulceration (VLU). Dermal fibrosis is the result of matrix metalloproteinase (MMP) activation and fibroblast senescence.
Recent studies by Sindrilaru et al. (2011) and Sindrilaru (2013) have identified a subset of fe-overloaded inflammatory M1-similar macrophages, which are implicated in the pathogenesis of CVD. Hb release from extravasated erythrocytes results in a serum haptoglobin/hemoglobin circuitous that is then taken upward by the macrophages, upon upregulation of the hemoglobin–haptoglobin receptor CD163. In CD163high macrophages, continuous uptake of Hb is thought to exist the crusade high intracellular concentrations of heme-atomic number 26 which induce an unrestrained pro-inflammatory macrophage activation. Macrophage iron can be farther increased during inflammation by virtue of increased systemic or local hepcidin expression, which leads to reduction in ferroportin, an fe efflux protein, resulting in intracellular iron aggregating. Individuals may also be predisposed to CVD disease through a genetic inability to counteract the skin iron overload (Caggiati et al., 2010). Studies have shown that common hemochromatosis cistron mutations such equally the C282Y mutation significantly increase the risk of ulcer in CVD by about seven times (Zamboni et al., 2005).
In hereditary hemochromatosis, plasma iron content increases beyond the iron bounden capacity of transferrin, although normal erythropoiesis is occurring. Studies using quantitative nuclear microscopy measurements of iron concentration in the epidermis (which is a readily accessible tissue) have shown that pare atomic number 26 levels reflect the liver iron overload. Interestingly, this technique has been proposed equally a clinical tool to enable better informed decisions on when to initiate, change or terminate phlebotomy therapy. In both CVD and hereditary hemochromatosis, parenchymal iron deposition leads to activation of metalloproteinases and subsequently fibrosis. Hereditary hemochromotosis has also been used to study the furnishings of iron on the aging process. Relative iron overload may also have a deleterious effect on normal pare aging, as iron chelators assist "successful" normal skin crumbling when applied topically (Polla et al., 2003).
Leg ulceration represents one of the main causes of morbidity in sickle cell anemia (SCA). Known risk factors for leg ulcer development in SCA include Hb (≤6 g/dL), lower levels of fetal Hb, hemolysis, raised lactate dehydrogenase (Lobe et al., 1992), infections and inflammation (Cumming et al., 2008). It is likely that in SCA and other disorders causing hemolytic anemia (such as hereditary spherocytosis, thalassemias, and other hemoglobinopathies) the deleterious furnishings of excessive local cutaneous or macrophage atomic number 26 degradation play key roles in poor wound healing.
Conclusion
Over recent years there has been some advocacy in knowledge most atomic number 26 in the pare and iron deficiency in cutaneous wound healing. It is articulate from studies on pathology of CVD that loftier atomic number 26 in macrophages tin can induce unrestrained proinflammatory macrophage activation. Furthermore in cases of iron deficiency/anemia of inflammation, when serum hepcidin levels are elevated, hepcidin/ferroportin interaction tin can lead to increased fe concentration in cells particularly macrophage and this could also have a detrimental effect on wound healing. Iron deficiency without inflammation is probable to affect 1 of the later stages of wound healing such equally remodeling. Boosted in-depth scientific study of both the underlying pathophysiological mechanisms and office of local cutaneous iron in conditions associated with iron overload and iron deficiency is a priority. Iron is a potential therapeutic target in the skin by awarding of topical iron chelators and other novel pharmacological agents, and in delayed cutaneous wound healing by treatment of iron deficiency.
Conflict of Involvement Argument
The authors declare that the inquiry was conducted in the absence of whatsoever commercial or fiscal relationships that could be construed as a potential conflict of interest.
References
Agarwal, Five., Sachdev, A., Singh, R., Lehl, S., and Basu, S. (2003). Autoimmune hemolytic anemia associated with beneficial ovarian cyst: a example study and review of literature. Indian J. Med. Sci. 57, 504–506.
Pubmed Abstract | Pubmed Full Text
Aroun, A., Zhong, J. L., Tyrrell, R. M., and Pourzand, C. (2012). Iron, oxidative stress and the example of solar ultraviolet a radiation. Photochem. Photobiol. Sci. 11, 118–134. doi: ten.1039/c1pp05204g
Pubmed Abstract | Pubmed Total Text | CrossRef Full Text
Bains, J. West., Crawford, D. T., and Ketcham, A. S. (1966). Effect of chronic anemia on wound tensile strength: correlation with blood volume, total red blood prison cell volume and proteins. Ann. Surg. 164, 243–246. doi: 10.1097/00000658-196608000-00009
Pubmed Abstruse | Pubmed Full Text | CrossRef Full Text
Bari, Grand. A., Sutradhar, Southward. R., Sarker, C. Northward., Ahmed, S., Miah, A. H., Alam, Chiliad. Chiliad.,et al. (2013). Cess of anaemia in patients with rheumatoid arthritis. Mymensingh Med. J. 22, 248–254.
Caggiati, A., Rosi, C., Casini, A., Cirenza, M., Petrozza, V., Acconcia, M. C.,et al. (2010). Skin atomic number 26 deposition characterises lipodermatosclerosis and leg ulcer. Eur. J. Vasc. Endovasc. Surg. 40, 777–782. doi: x.1016/j.ejvs.2010.08.015
Pubmed Abstract | Pubmed Total Text | CrossRef Full Text
Cumming, V., King, L., Fraser, R., Serjeant, G., and Reid, K. (2008). Venous incompetence, poverty and lactate dehydrogenase in Jamaica are important predictors of leg ulceration in sickle cell anaemia. Br. J. Haematol. 142, 119–125. doi: 10.1111/j.1365-2141.2008.07115.x
Pubmed Abstract | Pubmed Total Text | CrossRef Full Text
Donneys, A., Weiss, D. M., Deshpande, S. S., Ahsan, Due south., Tchanque-Fossuo, C. N., Sarhaddi, D.,et al. (2013). Localized deferoxamine injection augments vascularity and improves bony wedlock in pathologic fracture healing after radiotherapy. Os 52, 318–325. doi: 10.1016/j.bone.2012.10.014
Pubmed Abstract | Pubmed Total Text | CrossRef Full Text
Farberg, A. S., Jing, X. L., Monson, L. A., Donneys, A., Tchanque-Fossuo, C. N., Deshpande, S. S.,et al. (2012). Deferoxamine reverses radiations induced hypovascularity during bone regeneration and repair in the murine mandible. Bone l, 1184–1187. doi: 10.1016/j.bone.2012.01.019
Pubmed Abstract | Pubmed Total Text | CrossRef Full Text
Heughan, C., Grislis, G., and Hunt, T. 1000. (1974). The effect of anemia on wound healing. Ann. Surg. 179, 163–167. doi: 10.1097/00000658-197402000-00009
CrossRef Full Text
Hong, Westward. 10., Hu, M. South., Esquivel, M., Liang, G. Y., Rennert, R. C., McArdle, A.,et al. (2014). The role of hypoxia-inducible factor in wound healing. Adv. Wound Intendance (New Rochelle) 3, 390–399. doi: 10.1089/wound.2013.0520
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Isaacs, J. D., Harari, O., Kobold, U., Lee, J. Due south., and Bernasconi, C. (2013). Effect of tocilizumab on haematological markers implicates interleukin-6 signalling in the anaemia of rheumatoid arthritis. Arthritis Res. Ther. 15, R204. doi: 10.1186/ar4397
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Jacob, R. A., Sandstead, H. H., Munoz, J. M., Klevay, 50. Grand., and Milne, D. B. (1981). Whole body surface loss of trace metals in normal males. Am. J. Clin. Nutr. 34, 1379–1383.
Pubmed Abstruse | Pubmed Full Text
Jacobson, 1000. J., and Vanprohaska, J. (1965). The healing of wounds in iron deficiency. Surgery 57, 254–258.
Jonsson, K., Jensen, J. A., Goodson, W. H. Three, Scheuenstuhl, H., West, J., Hopf, H. W.,et al. (1991). Tissue oxygenation, anemia, and perfusion in relation to wound healing in surgical patients. Ann. Surg. 214, 605–613. doi: 10.1097/00000658-199111000-00011
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Khanbhai, M. Fifty. S., Wright, J. A., Hurel, S., and Richards, T. (2012). Anaemia, inflammation, renal function, and the diabetic foot: what are the relationships? Diabet. Human foot J. 15:eight.
Kurz, 1000., Steigleder, G. K., Bischof, W., and Gonsior, B. (1987). PIXE analysis in unlike stages of psoriatic skin. J. Investig. Dermatol. 88, 223–226. doi: 10.1111/1523-1747.ep12525385
CrossRef Total Text
Leveque, N., Robin, S., Makki, S., Muret, P., Rougier, A., and Humbert, P. (2003). Iron and ascorbic acrid concentrations in human dermis with regard to age and body sites. Gerontology 49, 117–122. doi: x.1159/000067951
Pubmed Abstruse | Pubmed Full Text | CrossRef Full Text
Lobe, T. East., Richardson, C. J., Boulden, T. F., Swischuk, L. E., Hayden, C. K., and Oldham, K. T. (1992). Mycotic thromboaneurysmal illness of the abdominal aorta in preterm infants: its natural history and its direction. J. Pediatr. Surg. 27, 1054–1059; discussion 9–60. doi: ten.1016/0022-3468(92)90559-P
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Macon, W. 50., and Pories, W. J. (1971). The upshot of iron deficiency anemia on wound healing. Surgery 69, 792–796.
Mohammadpour, M., Behjati, M., Sadeghi, A., and Fassihi, A. (2013). Wound healing by topical awarding of antioxidant iron chelators: kojic acid and deferiprone. Int. Wound J. ten, 260–264. doi: 10.1111/j.1742-481X.2012.00971.ten
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Oliveira Sampaio, S. C., de C. Monteiro, J. S., Cangussu, One thousand. C., Pires Santos, K. Chiliad., Dos Santos, Thou. A., Dos Santos, J. N.,et al. (2013). Effect of laser and LED phototherapies on the healing of cutaneous wound on healthy and iron-deficient Wistar rats and their touch on fibroblastic activity during wound healing. Lasers Med. Sci. 28, 799–806. doi: 10.1007/s10103-012-1161-ix
Pubmed Abstract | Pubmed Total Text | CrossRef Full Text
Pavlidis, T. Due east., Galatianos, I. North., Papaziogas, B. T., Lazaridis, C. Northward., Atmatzidis, K. S., Makris, J. G.,et al. (2001). Complete dehiscence of the abdominal wound and incriminating factors. Eur. J. Surg. 167, 351–354; discussion 5. doi: 10.1080/110241501750215221
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Pelle, E., Jian, J., Declercq, 50., Dong, Thou., Yang, Q., Pourzand, C.,et al. (2011). Protection confronting ultraviolet A-induced oxidative harm in normal human epidermal keratinocytes nether post-menopausal atmospheric condition by an ultraviolet A-activated caged-iron chelator: a pilot study. Photodermatol. Photoimmunol. Photomed. 27, 231–235. doi: ten.1111/j.1600-0781.2011.00604.ten
Pubmed Abstract | Pubmed Full Text | CrossRef Total Text
Phelps, G. R., Einhorn, T. A., Vigorita, V. J., Lundin, A. P., and Friedman, E. A. (1986). Fracture healing with deferoxamine therapy in a patient with aluminum-associated osteomalacia. ASAIO Trans. 32, 198–200.
Pubmed Abstract | Pubmed Full Text
Polefka, T. Chiliad., Bianchini, R. J., and Shapiro, S. (2012). Interaction of mineral salts with the skin: a literature survey. Int. J. Cosmet. Sci. 34, 416–423. doi: 10.1111/j.1468-2494.2012.00731.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Pouillot, A. P. A., and Polla, B. South. (2013). Iron and fe chelators: a review on potential effects on skin crumbling. Curr. Ageing Sci. 6, 225–231. doi: x.2174/18746098112059990037
CrossRef Total Text
Rayner, R. C. K., Keaton, J., Prentice, J., and Santamaria, Northward. (2009). Leg ulcers: atypical presentations and associated comorbidities. Wound Pract. Res. 17, 168–185.
Richardson, D. R., Dickson, L., and Baker, E. (1996). Intermediate steps in cellular iron uptake from transferrin. II. A cytoplasmic pool of iron is released from cultured cells via temperature-dependent mechanical wounding. In vitro cellular and developmental biology. Animal 32, 486–495. doi: 10.1007/BF02723052
CrossRef Total Text
Sandberg, N., and Zederfeldt, B. (1960). Influence of acute hemorrhage on wound healing in the rabbit. Acta Chir. Scand. 118, 367–371.
Pubmed Abstruse | Pubmed Full Text
Sindrilaru, A., and Scharffetter-Kochanek K. (2013). Disclosure of the culprits: macrophages-versatile regulators of wound healing. Adv. Wound Intendance (New Rochelle) 2, 357–368. doi: ten.1089/wound.2012.0407
Pubmed Abstruse | Pubmed Total Text | CrossRef Full Text
Sindrilaru, A P. T., Wieschalka, S., Baican, C., Baican, A., Peter, H., Hainzl, A.,et al. (2011). An unrestrained proinflammatory M1 macrophage population induced by fe impairs wound healing in humans and mice. J. Clin. Invest. 121, 985–997. doi: 10.1172/JCI44490
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Sukumaran, A., James, J., Janardhan, H. P., Amaladas, A., Suresh, L. M., Danda, D.,et al. (2013). Expression of iron-related proteins in the duodenum is up-regulated in patients with chronic inflammatory disorders. Br. J. Nutr. 111, 1059–1068. doi: x.1017/S0007114513003334
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Taylor, J. E., Laity, P. R., Hicks, J., Wong, S. Due south., Norris, Thousand., Khunkamchoo, P.,et al. (2005). Extent of fe pick-upwards in deforoxamine-coupled polyurethane materials for therapy of chronic wounds. Biomaterials 26, 6024–6033. doi: 10.1016/j.biomaterials.2005.03.015
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Waterman, D. F., Birkhill, F. R., Pirani, C. L., and Levenson, S. M. (1952). The healing of wounds in the presence of anemia. Surgery 31, 821–828.
Weinstein, G. Southward., Maves, Chiliad. D., and McCormack, M. 50. (1989). Deferoxamine decreases necrosis in dorsally based pig skin flaps. Otolaryngol. Head Neck Surg. 101, 559–561.
Pubmed Abstruse | Pubmed Full Text
Wenk, J., Foitzik, A., Achterberg, V., Sabiwalsky, A., Dissemond, J., Meewes, C.,et al. (2001). Selective option-upwardly of increased iron by deferoxamine-coupled cellulose abrogates the iron-driven induction of matrix-degrading metalloproteinase 1 and lipid peroxidation in human dermal fibroblasts in vitro: a new dressing concept. J. Invest. Dermatol. 116, 833–839. doi: x.1046/j.1523-1747.2001.01345.ten
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Zamboni, P., Tognazzo, S., Izzo, K., Pancaldi, F., Scapoli, G. 50., Liboni, A.,et al. (2005). Hemochromatosis C282Y cistron mutation increases the adventure of venous leg ulceration. J. Vasc. Surg. 42, 309–314. doi: 10.1016/j.jvs.2005.04.003
Pubmed Abstruse | Pubmed Full Text | CrossRef Full Text
Zhong, J. L., Yiakouvaki, A., Holley, P., Tyrrell, R. Chiliad., and Pourzand, C. (2004). Susceptibility of skin cells to UVA-induced necrotic cell decease reflects the intracellular level of labile atomic number 26. J. Invest. Dermatol. 123, 771–780. doi: ten.1111/j.0022-202X.2004.23419.ten
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Can Iron Be Absorbed Through Skin?,
Source: https://www.frontiersin.org/articles/10.3389/fphar.2014.00156/full
Posted by: holtthea1980.blogspot.com


0 Response to "Can Iron Be Absorbed Through Skin?"
Post a Comment